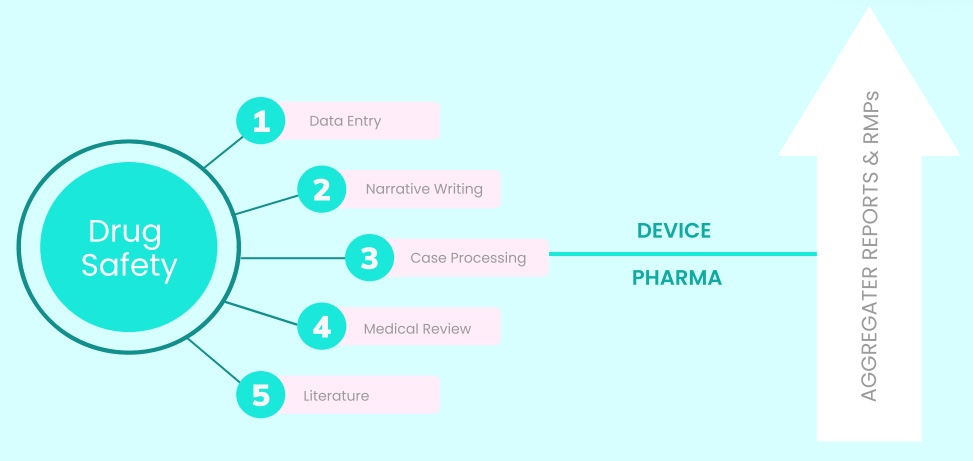
Flexible Suite of Drug Safety & Pharmacovigilance Services
Developing drugs and devices to manage human health is indeed a serious business
but developing safe drugs for mankind is the truth of the truths. Of all the steps,
ascertaining the safety of drug is the most crucial one as an unsafe drug can do more
harm to the body than the disease itself. So, it becomes most important for the
pharmaceutical company to identify the safety information about the drug/device in a
timely manner to avoid any future uncertainty. To do so and ensure the safety of
underdevelopment drug/device, the most essential step is to thoroughly collect as well
as analyse its safety information data. A well collected and analysed data is required
for the success of clinical research and to maintain the post-marketing drug licenses.
At Global Pharma Tek, we understand the criticality of patient safety, drug safety and
pharmacovigilance for every clinical research. We offer drug safety and
pharmacovigilance consulting services throughout the life cycle of a drug/device
development spanning from preclinical, clinical to post-marketing. Our team of
experienced drug safety and pharmacovigilance experts can assist you in evaluating
and managing the safety concerns of your product.