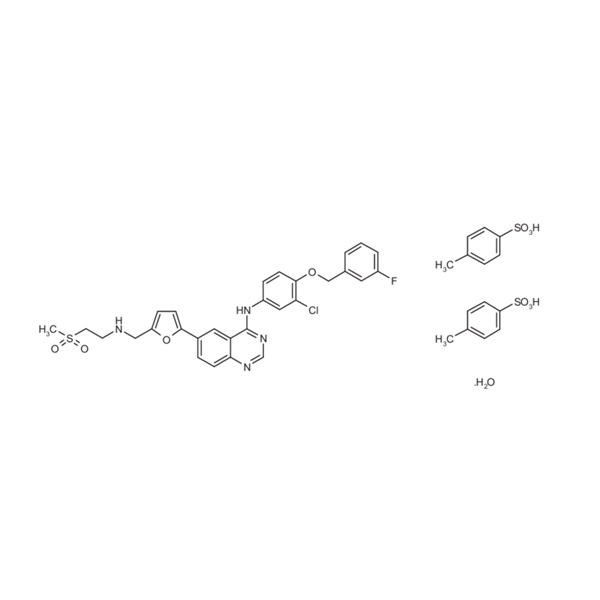
Lifitegrast API
CAS No.: 1025967-78-5 | GMP Certified
Global Pharma Tek offers Lifitegrast API, produced under GMP-certified environments with precise process controls to support ophthalmic formulation requirements.
The API demonstrates high purity, defined impurity specifications, and compatibility with advanced ocular drug delivery systems.
With integrated manufacturing and regulatory coordination, Global Pharma Tek delivers transparent documentation and dependable quality governance throughout lifecycle development.
Product Description
- Product Name: Lifitegrast
- Chemical Name: Lifitegrast
- CAS No.: 1025967-78-5
- Quality Grade: USP / EP
- Therapeutic Category: Ophthalmology / Dry eye disease
- DMF Support: Available on request
- Compliance: GMP Certified
- Availability: Campaign-based production
- For regulatory files or formulation support: info@globalpharmatek.com
Product Enquiry
Recommended Products

Alogliptin CAS No. : 850649-62-6
View Details
Bilastine CAS No. : 202189-78-4
View Details
Ciprofloxacin CAS No. : 85721-33-1
View Details
Clopidogrel Hydrogen Sulfate Ph. Eur. CAS No. : 113665-84-2
View Details
Fexofenadine HclPh.Eur. CAS No. : 138452-21-8
View Details
Flurbiprofen EP CAS No. : 5104-49-4
View Details
Gliclazide CAS No. : 21187-98-4
View Details
Hydroxy Chloroquine CAS No. : 118-42-3
View Details
Irbesartan Usp Form A CAS No. : 138402-11-6
View Details
Losartan Pottasium CAS No. : 124750-99-8
View Details
Telmisartan EP CAS No. : 144701-48-4
View Details
Azithromycin CAS No. : 83905-01-5
View Details
Pregabalin CAS No. : 148553-50-8
View Details
Paracetamol CAS No. : 103-90-2
View Details
