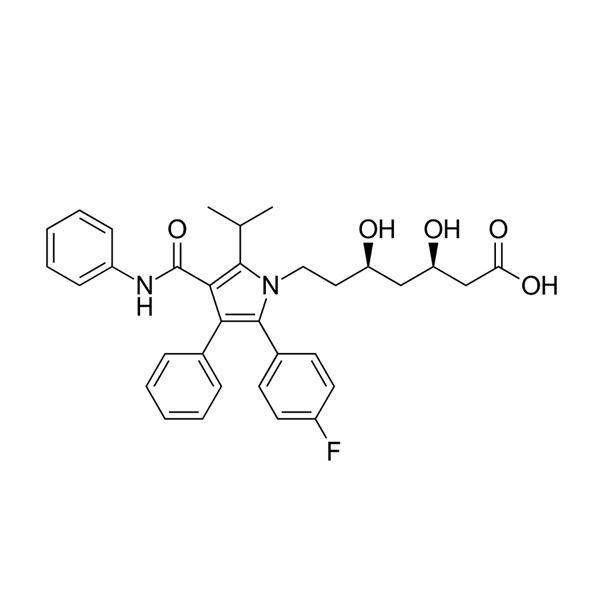
Atorvastatin API
CAS No.: 134523-00-5 | GMP Certified
Global Pharma Tek offers Atorvastatin API, produced in GMP-certified systems with robust quality controls suitable for large-scale cardiovascular drug manufacturing.
The API is known for its consistent physicochemical properties, stability, and suitability for high-volume oral solid dosage formulations.
Leveraging integrated manufacturing infrastructure and regulatory support, Global Pharma Tek ensures reliable documentation and sustained quality management.
Product Description
- Product Name: Atorvastatin
- Chemical Name: Atorvastatin
- CAS No.: 134523-00-5
- Quality Grade: USP / EP
- Therapeutic Category: Lipid-lowering agent / Cardiovascular
- DMF Support: Available on request
- Compliance: GMP Certified
- Availability: Commercial supply
- For pricing, documentation, or supply inquiries: info@globalpharmatek.com
Product Enquiry
Recommended Products

Alogliptin CAS No. : 850649-62-6
View Details
Bilastine CAS No. : 202189-78-4
View Details
Ciprofloxacin CAS No. : 85721-33-1
View Details
Clopidogrel Hydrogen Sulfate Ph. Eur. CAS No. : 113665-84-2
View Details
Fexofenadine HclPh.Eur. CAS No. : 138452-21-8
View Details
Flurbiprofen EP CAS No. : 5104-49-4
View Details
Gliclazide CAS No. : 21187-98-4
View Details
Hydroxy Chloroquine CAS No. : 118-42-3
View Details
Irbesartan Usp Form A CAS No. : 138402-11-6
View Details
Losartan Pottasium CAS No. : 124750-99-8
View Details
Telmisartan EP CAS No. : 144701-48-4
View Details
Azithromycin CAS No. : 83905-01-5
View Details
Pregabalin CAS No. : 148553-50-8
View Details
Paracetamol CAS No. : 103-90-2
View Details
