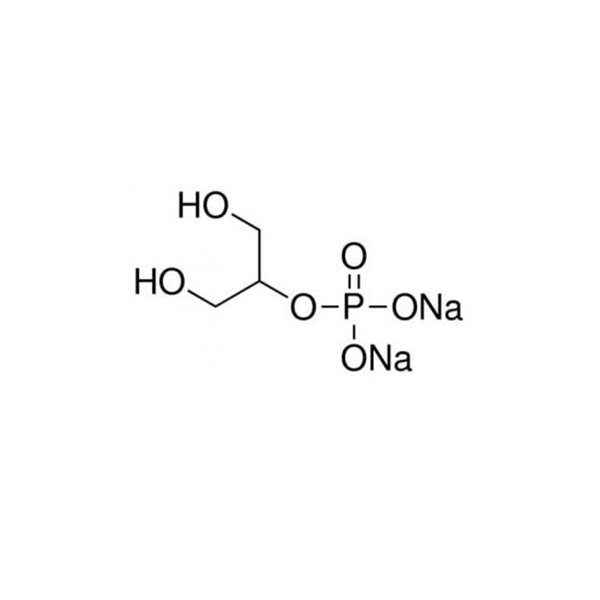
Sodium Glycerophosphate EP
CAS No.: 13408-09-8 | GMP Certified
Global Pharma Tek offers Sodium Glycerophosphate EP, produced in GMP-certified environments to meet parenteral and nutritional formulation standards.
The API is known for its defined composition, stability profile, and suitability for electrolyte supplementation.
Through coordinated manufacturing and regulatory frameworks, Global Pharma Tek ensures transparent technical documentation and quality oversight.
Product Description
- Product Name: Sodium Glycerophosphate
- Chemical Name: Sodium glycerophosphate
- CAS No.: 13408-09-8
- Quality Grade: EP
- Therapeutic Category: Electrolyte / Nutritional API
- Compliance: GMP Certified
- Availability: Commercial supply
- For batch schedules or technical documentation: info@globalpharmatek.com
Product Enquiry
Recommended Products

Alogliptin CAS No. : 850649-62-6
View Details
Bilastine CAS No. : 202189-78-4
View Details
Ciprofloxacin CAS No. : 85721-33-1
View Details
Clopidogrel Hydrogen Sulfate Ph. Eur. CAS No. : 113665-84-2
View Details
Fexofenadine HclPh.Eur. CAS No. : 138452-21-8
View Details
Flurbiprofen EP CAS No. : 5104-49-4
View Details
Gliclazide CAS No. : 21187-98-4
View Details
Hydroxy Chloroquine CAS No. : 118-42-3
View Details
Irbesartan Usp Form A CAS No. : 138402-11-6
View Details
Losartan Pottasium CAS No. : 124750-99-8
View Details
Telmisartan EP CAS No. : 144701-48-4
View Details
Azithromycin CAS No. : 83905-01-5
View Details
Pregabalin CAS No. : 148553-50-8
View Details
Paracetamol CAS No. : 103-90-2
View Details
